What’s the deal with nitrogen?
Hobbyists often become confused about the various methods of measuring nitrogen(N) in the planted aquarium. Plants utilize nitrogen in order to grow, and the plant is able to extract this nitrogen from several other types of substances in the aquarium, including ammonia, urea, nitrate, and many others. However, hobbyists often become used to only testing for nitrate and ignoring all other sources of nitrogen, mostly because nitrate is the easiest substance to dose and test in the aquarium. As hobbyists become more knowledgeable about nitrogen sources in the planted tank, accounting for nitrogen sources other than nitrate in supplementation becomes more and more important.
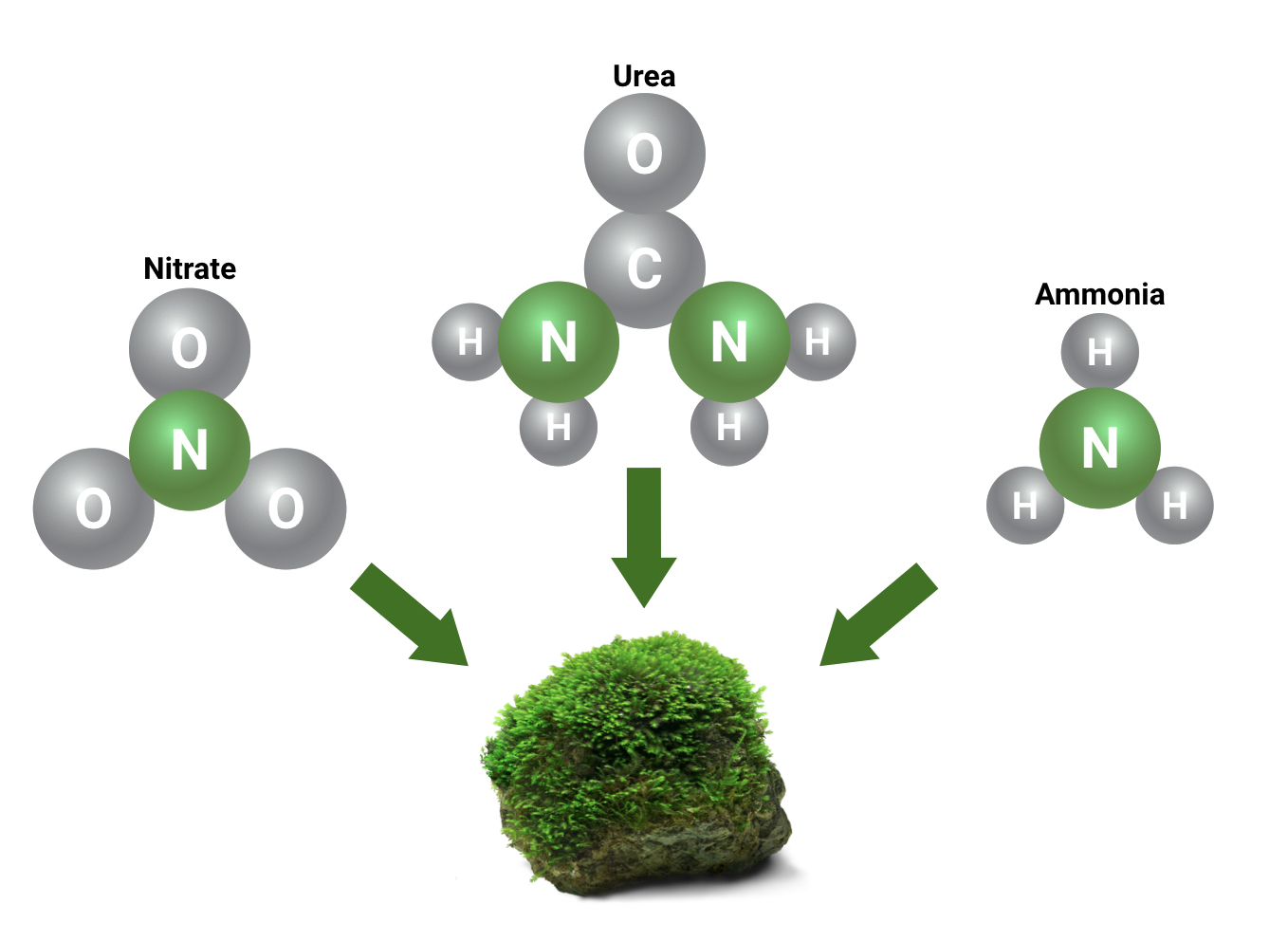
What are these measurements?
If you regularly measure nitrate (or any other major water parameter), you are probably used to seeing “ppm” or “mg/L” as a unit of measure. It is important to remember that, despite some confusing naming conventions, these units are both measurements of weight per volume. This could be compared to a unit of measure like “grams per gallon” or “pounds per cubic foot”. In fishkeeping, mg/l is “milligrams of substance per liter of water”, and ppm is “milligrams of substance per kilogram of water" (with the assumption that 1 liter weighs exactly a kilogram). These units are calculated the same way, and so are considered to be more-or-less equal.
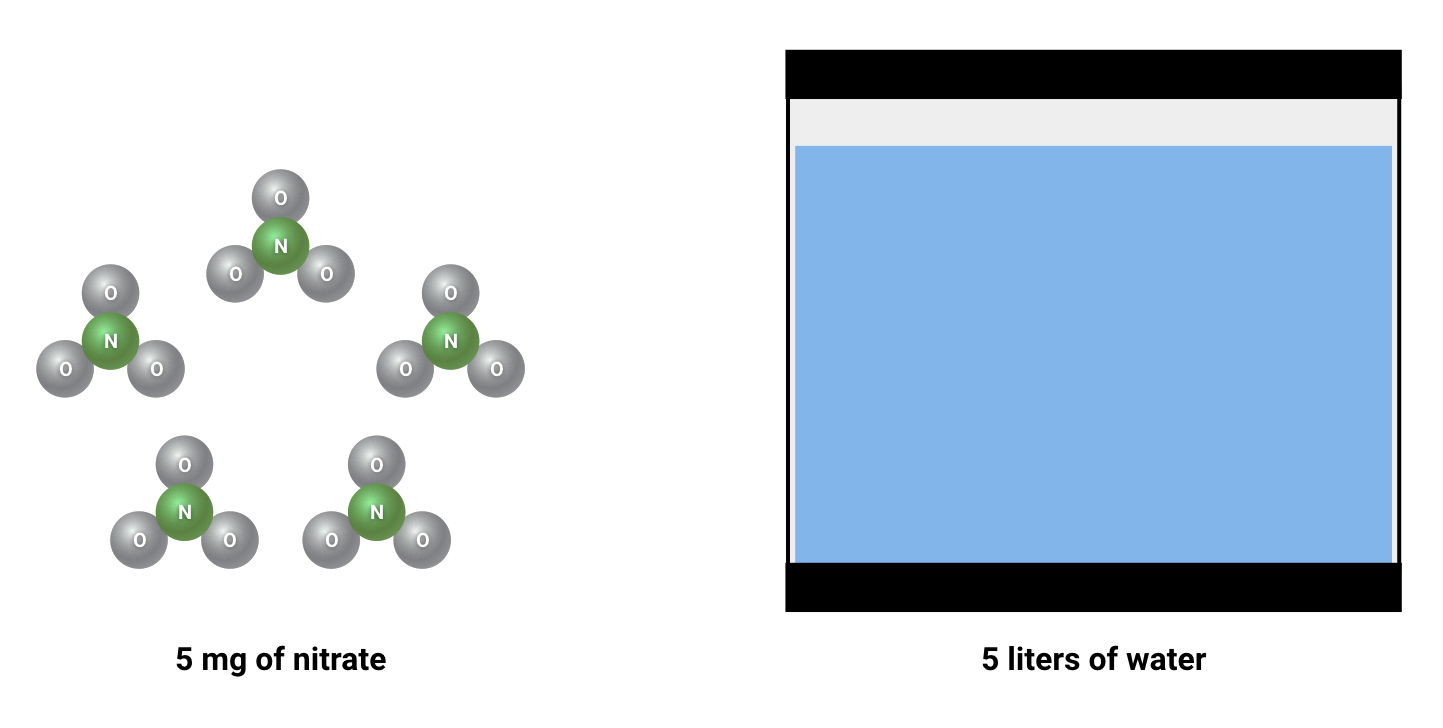
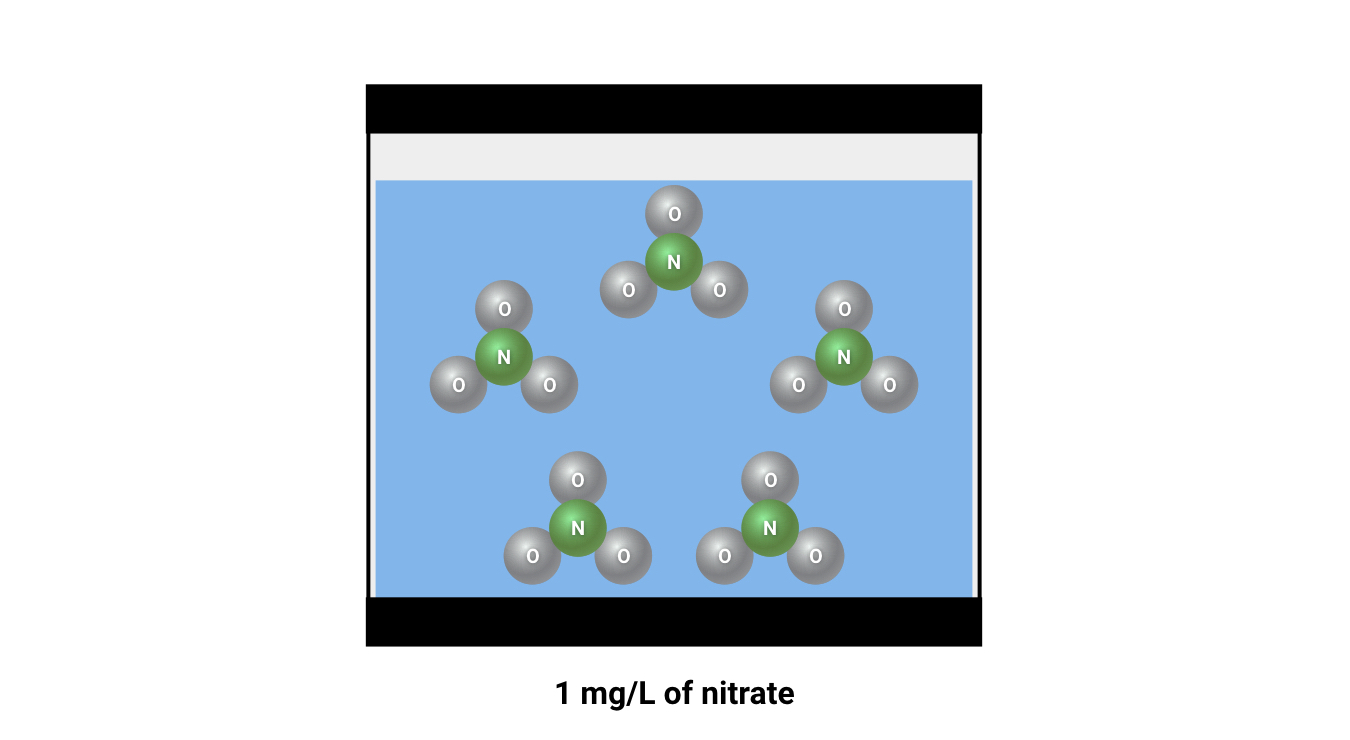
Why does the definition of “mg/L” matter for nitrogen dosing?
This matters because nitrogen(N) doesn’t usually hang out as a free and independent atom in the aquarium. It is always bound to something else. It could be bound to nitrate, urea, ammonia, or many other types of molecules. These molecules contain more atoms than just nitrogen, and these extra atoms add weight to the substance. If you are shooting for a specific concentration of nitrogen, you’ll need to account for the fact that the substances you are dosing are not 100% nitrogen.
Let’s take nitrate for example. We’ll be simplifying the weights of the various atoms for the sake of easy math:
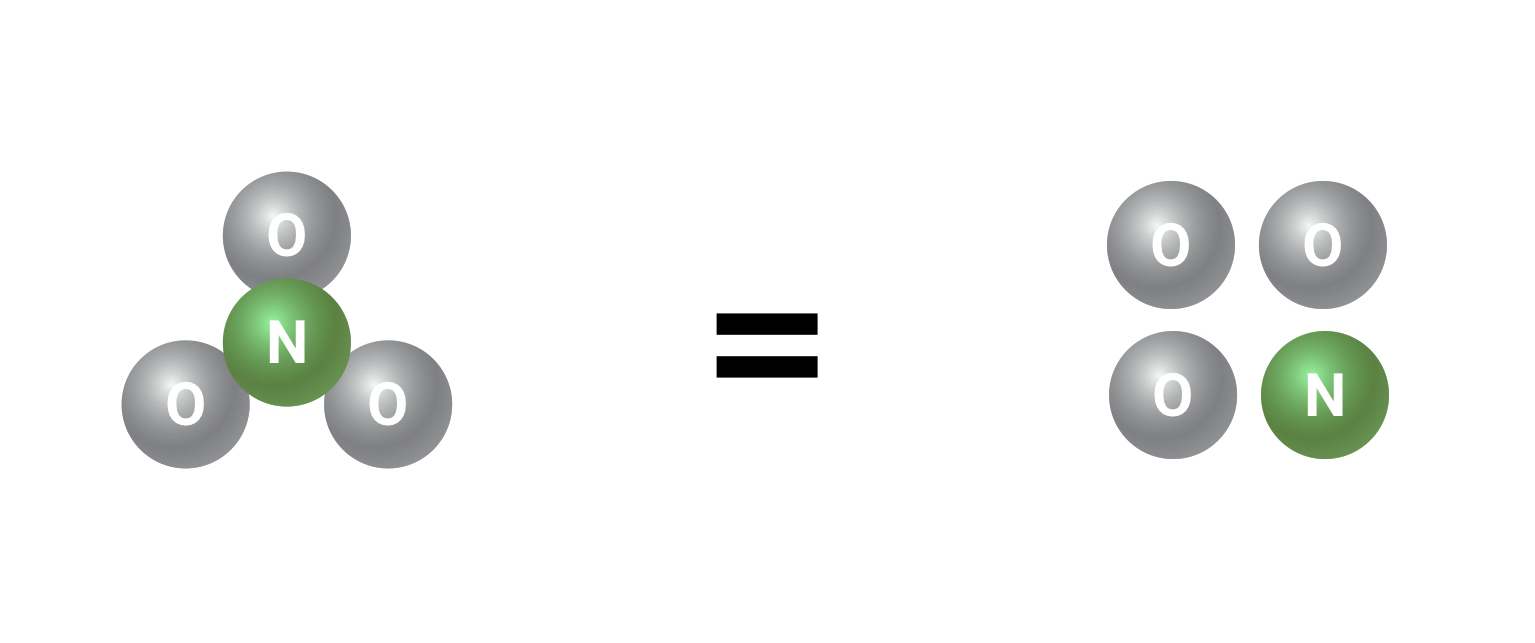
Nitrate is NO3. That’s three oxygen atoms and one nitrogen atom. The oxygen atoms take up about 3/4 of the weight of the nitrate molecule while the nitrogen takes up about 1/4 of the weight (this works because oxygen and nitrogen atoms weigh about the same amount).
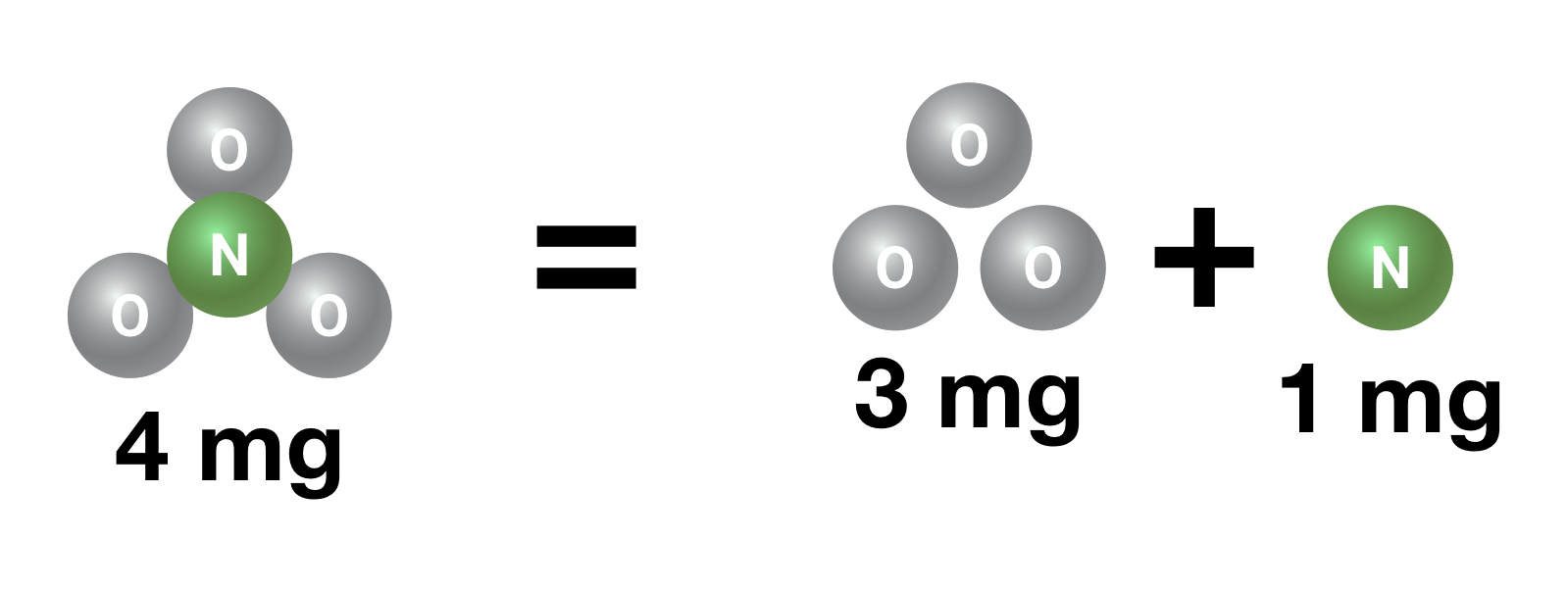
That means that 4 mg of nitrate contains about 1 mg of nitrogen and 3 mg of oxygen.
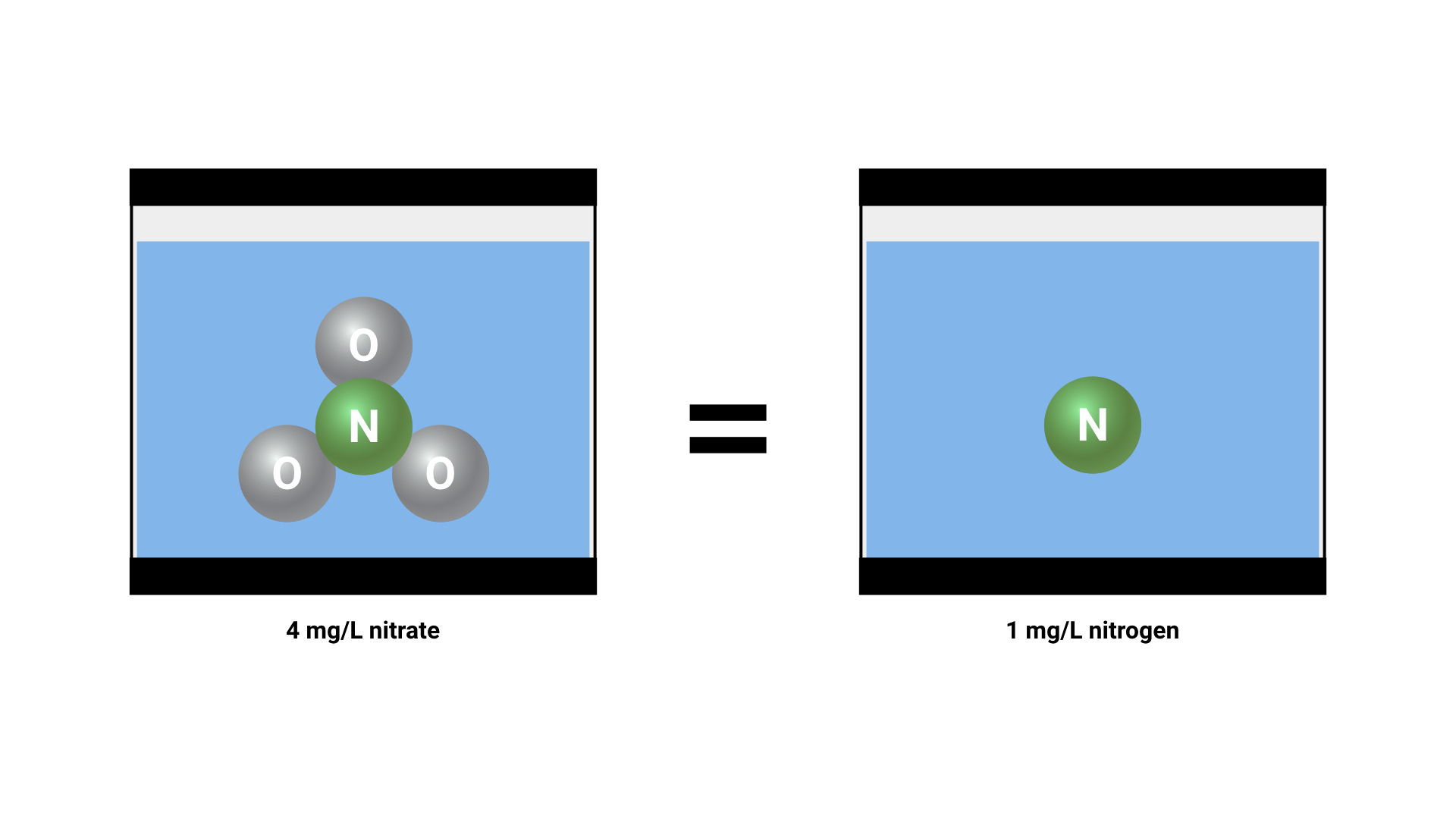
If we convert that into units of measure for concentrations in the aquarium, we can say that “4 mg/L of nitrate(NO3) contains the same amount of nitrogen as 1 mg/L of pure nitrogen(N)”
Fascinating chemistry lesson. Why does this matter for my plants?
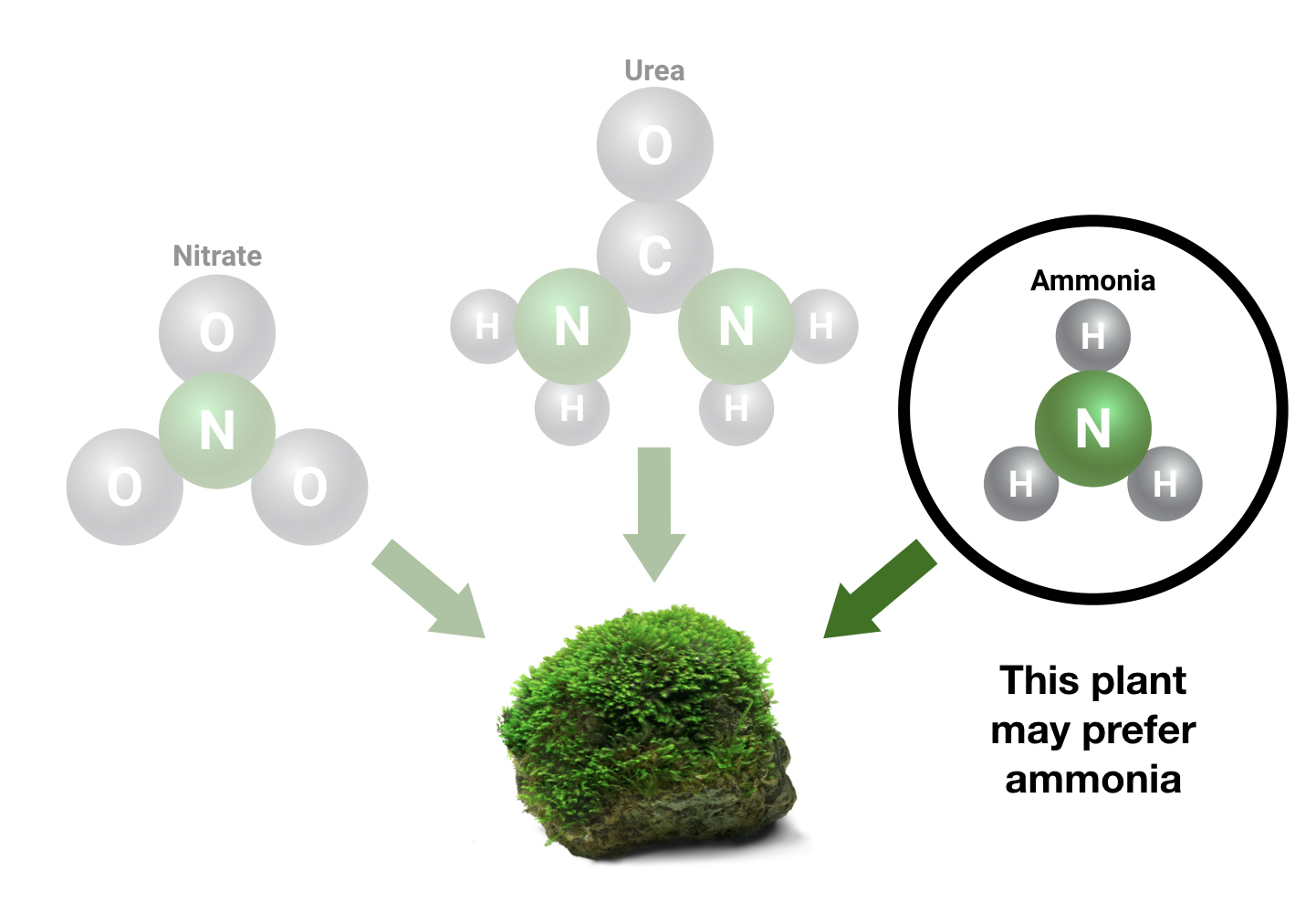
Your plants are absorbing nitrate, ammonia, urea, and other substances for the specific purpose of extracting the nitrogen. Some plants prefer ammonia, some prefer urea, and some prefer nitrate. Many sources will recommend to aim for around 10-20 mg/L (ppm) nitrate in the aquarium to ensure that the plants have plenty of nitrogen. This advice ignores the presence of sources of nitrogen other than nitrate for the sake of simplicity, which is good for new fishkeepers, but can be confusing for more advanced fishkeepers who know that plants do best if they have several types of nitrogen-containing molecules to choose from.
When dosing a product that contains multiple forms of nitrogen like Flourish Nitrogen™, it’s important to understand that not all of the nitrogen you are dosing will show up on a nitrate test kit. Our instructions state that “The beginner dose raises nitrogen by the same degree that 1 mg/L nitrate would”. Essentially, 2.5 mL of Flourish Nitrogen™ added to 40 gallons of water will add the same amount of nitrogen as 1 mg/L of nitrate. However, if you test your nitrate immediately after dosing Flourish Nitrogen™, you will get a reading of only 0.5 mg/L because half of the nitrogen in Flourish Nitrogen™ is bound up as nitrate and the other half is bound up as urea.
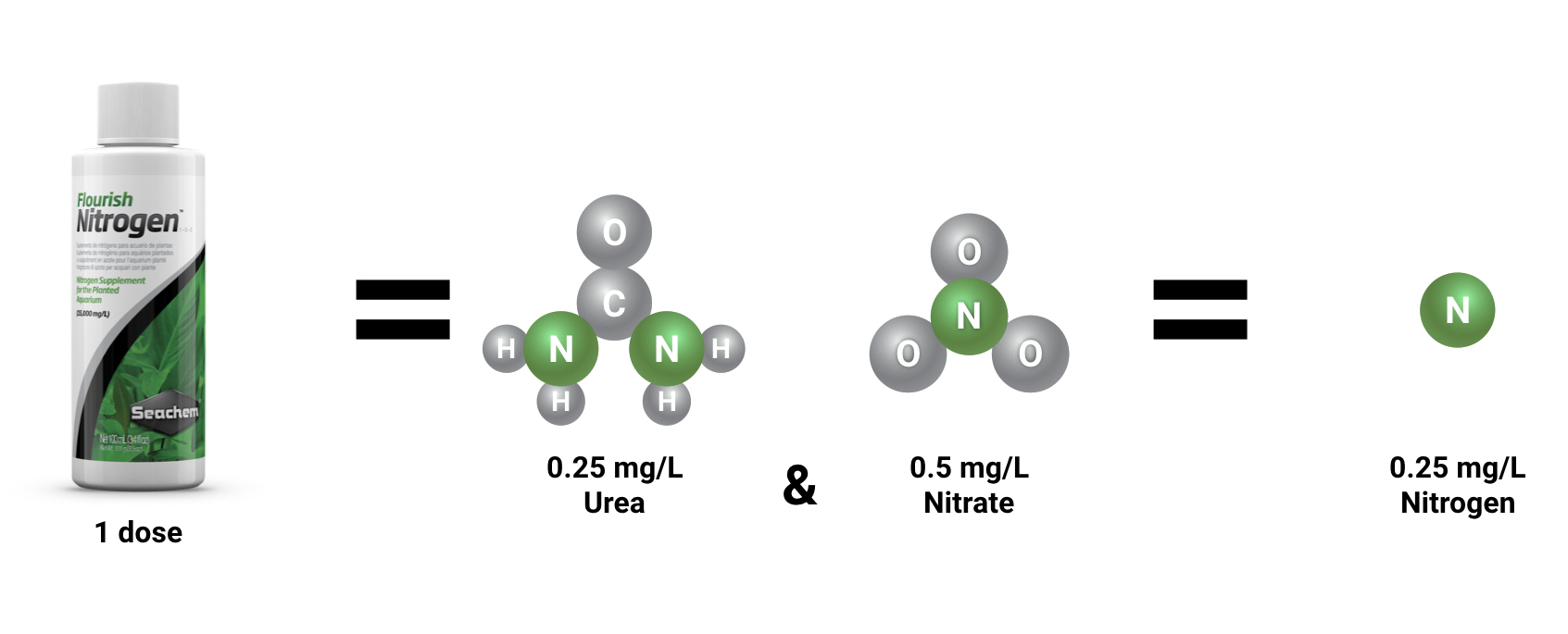
So, one dose of Flourish Nitrogen™ increases nitrogen (N) by 0.25 mg/L which is the same amount of nitrogen that you would get from 1 mg/L of nitrate. We call this a “nitrate equivalent”. If you are just testing and dosing your aquarium to reach a specific nitrate level, you should dose as if each addition of Flourish Nitrogen™ increases nitrate by 1 mg/L.
0 Comments